CorriX
Validity
A human study involving 140 adults in Germany confirmed
that CorriX consumption increases TFα IgM antibody levels.
 [Beneficial Microbes, 2016; 7(4): 485-500]
[Beneficial Microbes, 2016; 7(4): 485-500]
-
Overview of Human study
-
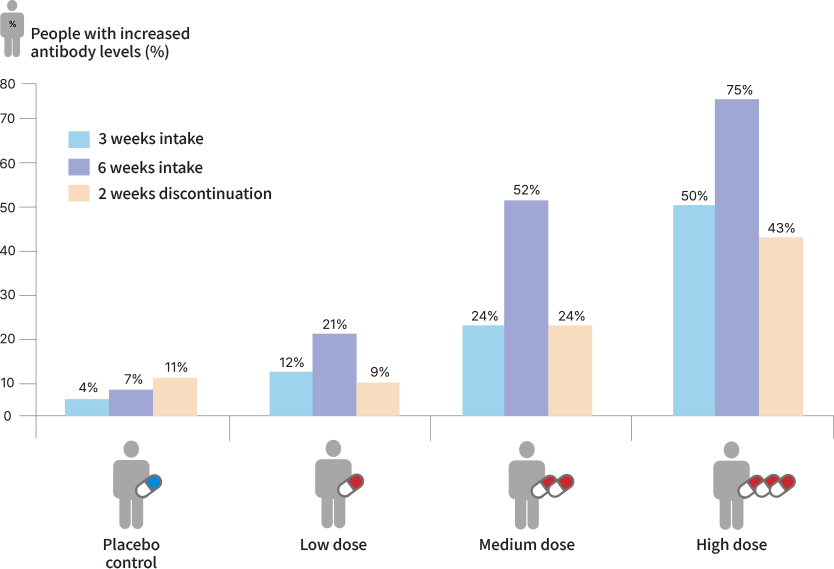
Result of Human study
Results
- A study involving 140 healthy adults confirmed that TFα IgM antibody levels increased after 6 weeks of CorriX consumption. However, it was also observed that TFα IgM antibody levels decreased after a 2-week discontinuation of consumption.
- The TFα antigen present on the surface of CorriX has been confirmed to be recognized by dendritic cells, triggering the production of TFα antibodies through a B-cell immune response mechanism.
- In conclusion, the human study demonstrated that CorriX plays a key role in promoting the natural production of TFα IgM antibodies in the body.
Value
The human study results demonstrates that increase in TFα IgM antibodies provides crucial scientific evidence supporting CorriX as a functional microbiome that contributes to the immune system in the body.
Additionally, these findings serve as a reliable scientific basis for the development of enhancing immunity functional products, further enhancing the scientific credibility and market value of CorriX-based products.
 [Beneficial Microbes, 2016; 7(4): 485-500]
[Beneficial Microbes, 2016; 7(4): 485-500]
Validation - Human study
- Validation of efficacy in humans
- Efficacy Validation in Humans Increased the level of
TFα antibodies in the body confirmed with CorriX consumption.

