Business
Business Area - Domestic
Domestic
- Supplying CorriX Raw material
- Functional Food Ingredients
Overseas
- Development of Global Functional Foods
- Approval for US Dietary Supplements
Medifood
- Human study for Cancer Patients
- Medifood for cancer patients in globe
Approval as
a Novel Food Ingredient
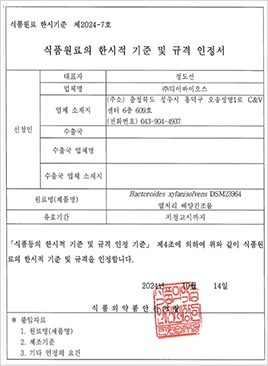
CorriX has been approved as a safe novel food ingredient by the Ministry of Food and Drug Safety (MFDS)
The approval system for novel food materials evaluates the safety of new food ingredients that have not been previously consumed domestically, recognizing them as food ingredients. This process demands thorough scientific evidence to validate the safety of the materials.
CorriX has already been validated as safe based on scientific evidence under the US FDA GRAS and EU EFSA Novel Food regulations. Additionally, it has been approved as safe by the Korean Ministry of Food and Drug Safety (MFDS) through a stringent verification process. The approval of CorriX as a safe novel food ingredient is one of the few cases in Korea where such approval has been granted.
The MFDS approval significantly enhances consumer trust in the safety of CorriX, while its application as a new ingredient across various food products unlocks extensive market potential in the domestic market.
Furthermore, this approval opens doors for collaboration with diverse companies and research institutions, paving the way for joint research and the development of new products. These opportunities are expected to generate synergy effects and drive innovation, underscoring the importance of CorriX in the food and functional food industries.
The approval system for novel food materials evaluates the safety of new food ingredients that have not been previously consumed domestically, recognizing them as food ingredients. This process demands thorough scientific evidence to validate the safety of the materials.
CorriX has already been validated as safe based on scientific evidence under the US FDA GRAS and EU EFSA Novel Food regulations. Additionally, it has been approved as safe by the Korean Ministry of Food and Drug Safety (MFDS) through a stringent verification process. The approval of CorriX as a safe novel food ingredient is one of the few cases in Korea where such approval has been granted.
The MFDS approval significantly enhances consumer trust in the safety of CorriX, while its application as a new ingredient across various food products unlocks extensive market potential in the domestic market.
Furthermore, this approval opens doors for collaboration with diverse companies and research institutions, paving the way for joint research and the development of new products. These opportunities are expected to generate synergy effects and drive innovation, underscoring the importance of CorriX in the food and functional food industries.
Next step - Approval as a Functional
Food Ingredient
CorriX aims for approval as a functional food ingredient for immune enhancement, supported by clinical data demonstrating its efficacy to increase TFα antibody levels.
Functional food ingredients are those that have been validated for safety and functionality by the Ministry of Food and Drug Safety (MFDS), providing consumers with high confidence in the approved functionality of the ingredient.
In the domestic immune-boosting health functional food market, demand has been increasing annually, reaching approximately KRW 1 trillion as of 2022 (Source: Korea Health Functional Food Association). CorriX aims to position itself as a specialized immune health brand by obtaining approval as a functional food ingredient.
Functional food ingredients are those that have been validated for safety and functionality by the Ministry of Food and Drug Safety (MFDS), providing consumers with high confidence in the approved functionality of the ingredient.
In the domestic immune-boosting health functional food market, demand has been increasing annually, reaching approximately KRW 1 trillion as of 2022 (Source: Korea Health Functional Food Association). CorriX aims to position itself as a specialized immune health brand by obtaining approval as a functional food ingredient.

