Business
Business Area-Overseas
Status of Overseas Regulatory Approvals
CorriX has been authorised by the U.S. FDA GRAS and the EU EFSA Novel Food as a safe novel food ingredient.
‘GRAS (Generally Recognized As Safe)’ is a system officially approved by the FDA for food ingredients deemed safe for consumption. Similarly, ‘Novel Food’ is a system established by EFSA to evaluate the safety of new food ingredients not consumed in Europe before 1997.
The GRAS and Novel Food notifications require thorough verification based on toxicity test data and clinical study results related to safety. CorriX has successfully undergone these rigorous evaluations by leading global institutions, making it a safe food ingredient for various applications.
The proven safety of CorriX provides consumers with confidence and establishes its competitive edge in the food market. With its high marketability as a novel food ingredient, CorriX holds significant value, facilitating its expansion into global markets.
‘GRAS (Generally Recognized As Safe)’ is a system officially approved by the FDA for food ingredients deemed safe for consumption. Similarly, ‘Novel Food’ is a system established by EFSA to evaluate the safety of new food ingredients not consumed in Europe before 1997.
The GRAS and Novel Food notifications require thorough verification based on toxicity test data and clinical study results related to safety. CorriX has successfully undergone these rigorous evaluations by leading global institutions, making it a safe food ingredient for various applications.
The proven safety of CorriX provides consumers with confidence and establishes its competitive edge in the food market. With its high marketability as a novel food ingredient, CorriX holds significant value, facilitating its expansion into global markets.
US FDA GRAS
notification
notification

European Union EFSA
Novel Food
Novel Food

Opinion from
FDA GRAS Letter Expert
The U.S. FDA GRAS letter for CorriX states that experts panels recognize CorriX as a potential candidate for dietary supplement (Structure/Function Claim)
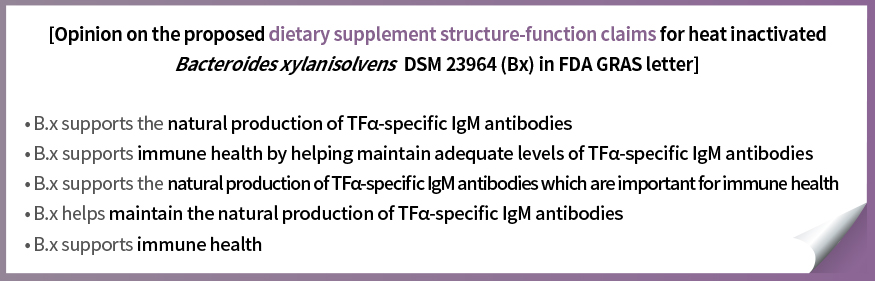
The related letter is a key data proves that CorriX can help immune health by naturally producing TFα antibodies in the body, based on the human study. It serves as important evidence to support the functionality of CorriX when registering with the U.S. FDA NDIN.
CorriX aims for the U.S. FDA NDI Notification and plans to enter the US dietary supplement market through Structure/Function Claims after registering as FDA NDI based on the expert opinions and human study results contained in the FDA GRAS letter.
CorriX aims for the U.S. FDA NDI Notification and plans to enter the US dietary supplement market through Structure/Function Claims after registering as FDA NDI based on the expert opinions and human study results contained in the FDA GRAS letter.
Overview of the US FDA Approval System
The U.S. FDA, thoroughly manages the functionality of dietary supplements, strictly regulates the safety and efficacy of products to ensure that only high-quality products that consumers can trust enter the market.
If a dietary supplement product claims a specific functionality, the FDA reviews the claims of structure/function (Structure/Function Claims) to approve that the functional information to be delivered to consumers is based on scientific evidence. In addition, products containing new ingredients must be registered as New Dietary Ingredients (NDIs) to prove safety and functionality.
To enter the U.S. dietary supplement market, it is significant to pass through the various approval hurdles. FDA approval serves as an important trust factor because it means that the product has sufficient scientific basis and data for its functionality. Acquiring FDA functional approval can provide confidence in the product to both consumers and distributors. It can also be foundation for strengthening global distribution networks and partnerships, as it facilitates entry into not only the U.S. market but also many countries that follow FDA standards.
If a dietary supplement product claims a specific functionality, the FDA reviews the claims of structure/function (Structure/Function Claims) to approve that the functional information to be delivered to consumers is based on scientific evidence. In addition, products containing new ingredients must be registered as New Dietary Ingredients (NDIs) to prove safety and functionality.
To enter the U.S. dietary supplement market, it is significant to pass through the various approval hurdles. FDA approval serves as an important trust factor because it means that the product has sufficient scientific basis and data for its functionality. Acquiring FDA functional approval can provide confidence in the product to both consumers and distributors. It can also be foundation for strengthening global distribution networks and partnerships, as it facilitates entry into not only the U.S. market but also many countries that follow FDA standards.
Explanation of Terms
* Dietary Supplement
- A product designed for diet supplement, which may include substances such as vitamins, minerals, herbs, amino acids, and enzymes.
- It supplements nutrients that may be lacking in a daily diet and provides consumers with safety and reliability based on scientific data through FDA regulation.
* Structure/Function Claim
- A structure/function claim indicates that a specific ingredient in a dietary supplement supports the structure or function of the human body. This claim can be displayed on dietary supplement products after undergoing FDA review based on scientific data.
- It helps consumers seeking immune health products choose the right product and better understand its effects.
* New Dietary Ingredient Notification
- It is a process of submitting a pre-market notification to the FDA to obtain approval for the safety of a new dietary ingredient when applying it to a dietary supplement.
- The New Dietary Ingredient Notification (NDIN) verifies the safety of the ingredient based on scientific evidence, ensuring consumer confidence and serving as an essential foundation for entering the U.S. market.
The importance of FDA approval to enter global market
-
Expansion based on trust
FDA approval officially guarantees the functionality and safety of food ingredients to global consumers, ensuring that CorriX is recognized as a trustworthy, high-quality product. CorriX can secure a high level of scientific credibility within the market by that. -
Easy entry into global markets
FDA approval, enables CorriX to more easily overcome regulatory barriers in various countries, serves as a key foundation for smooth entry into global markets. FDA approval serves as a standard for quality and safety not only in the US but also in other countries, making it an essential element for expanding into global markets. -
Strengthening premium brand image
FDA approval, highlights CorriX as a premium product with high quality and differentiation, serves as a stepping stone to secure strong competitiveness in the global market. By this certifications, CorriX is able to establish itself as a premium brand in the immune health functional products market, delivering deeper trust and value to consumers.
Strategy of Global Market Entry
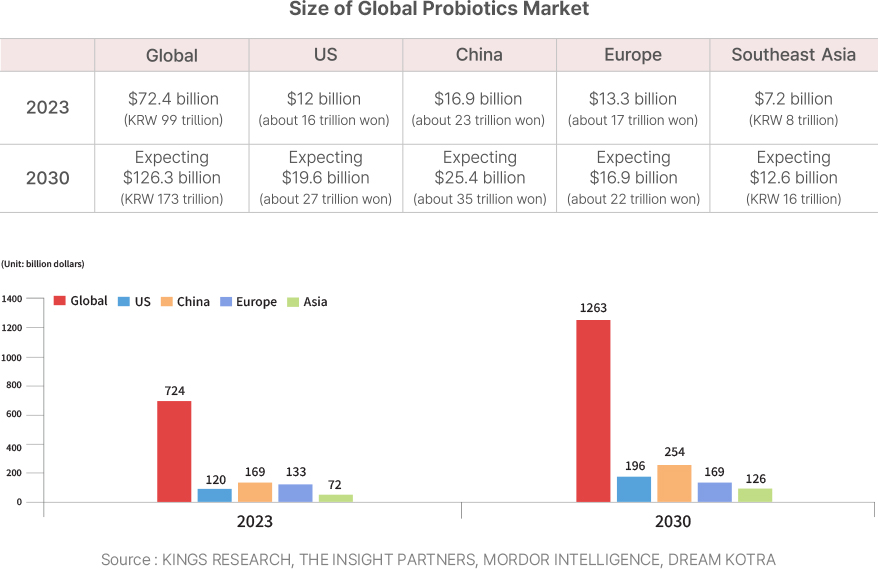



Global Market Strategy
CorriX aims for growth based on trust and competitiveness in the global market.

